Toxicology-Chemical Nature of Toxicants & Biochemical Transformations
Toxicant
Poison or toxicant is a substance that is harmful to living organisms.
A toxicant is a chemical compound that has an effect on organisms. Toxicants are typically introduced into the environment by human activity.
Physical forms of toxicants:
· Gases
· Vapors
· Dusts
· Fumes mists
Exposure:
· Dose
· toxicant concentration
· duration
· frequency
· rate
Toxicology
Toxicology is the study of the adverse effects of a toxicant on living organisms.
Toxicology is an applied science that incorporates biology, chemistry, physiology, pathology, physics, statistics, and sometimes immunology or ecology to help solve problems in forensic medicine, clinical treatments, pharmacy and pharmacology, public health, industrial hygiene, veterinary science, agriculture, and more, as well as giving basic insight into how an organism functions.
Toxicological Chemistry
Toxicological Chemistry is defined as the chemistry of toxic substances with emphasis upon their interactions with biologic tissue and living systems. In order to comprehend this topic, it is first necessary to have an appreciation of the chemical nature of inorganic and organic chemicals. An understanding of biochemistry is required to comprehend the ways in which xenobiotic substances in the body undergo biochemical processes and, in turn, affect these processes. An understanding biochemistry of toxicants requires an appreciation of Environmental chemistry.
Chemical Nature of Toxicants
It is not possible to exactly define a set of chemical characteristics that make a chemical species toxic. This is because of the large variety of ways in which substances can interact with substances, tissues, and organs to cause a toxic response. Because of subtle differences in their chemistry and biochemistry, similar substances may vary enormously in the degrees to which they cause a toxic response. For example, consider the toxic effects of carbon tetrachloride, CCl4 , a chemically closely related chlorofluorocarbon, dichlorofluoromethane, CCl2F2. Both of these compounds are completely halogenated derivatives of methane possessing very strong carbon-halogen bonds. As carbon tetrachloride is considered to be dangerous enough to have been banned from consumer products in 1970. It causes a large variety of toxic effects in humans, with chronic liver injury being the most prominent. Dichlorodifluoromethane, a Freon compound, is regarded as nontoxic, except for its action as a simple asphyxiant and lung irritant at high concentrations.
An increasingly useful branch of toxicological chemistry is the one dealing with quantitative structure-activity relationships. By relating the chemical structure and physical characteristicsof various compounds to their toxic effects, it is possible to predict the toxicologicaleffects of other compounds and classes of compounds.
With the qualification that there are exceptions to the scheme, it is possible to place toxic substances into several main categories. These are listed below:
Substances that exhibit extremes of acidity, basicity, dehydrating ability, or oxidizing power.
Examples include concentrated sulfuric acid -a strong acid with a tendency to dehydrate tissue,
strongly basic sodium hydroxide, and oxidant elemental fluorine, F2. Such species tend to be
nonkinetic poisons (see Section 6.9) and corrosive substances that destroy tissue by massively
damaging it at the site of exposure.
• Reactive substances that contain bonds or functional groups that are particularly prone to react
with biomolecules in a damaging way. One reason that diethyl ether, (C2H5)–O–(C2H5), is relatively
nontoxic is because of its lack of reactivity resulting from the very strong C–H bonds in the ethyl
groups and the very stable C–O–C ether linkage. A comparison of allyl alcohol with 1-propanol
(structural formulas below)
shows that the former is a relatively toxic irritant to the skin, eyes, and respiratory tract that also
damages liver and kidneys, whereas 1-propanol is one of the less toxic organic chemicals with an
LD50 (see Section 6.5) about 100 times that of allyl alcohol. As shown by the structures, allyl
alcohol differs from 1-propanol in having the relatively reactive alkenyl group C=C.
• Heavy metals, broadly defined, contain a number of members that are toxic by virtue of their
interaction with enzymes, tendency to bond strongly with sulfhydryl (–SH) groups on proteins,
and other effects.
• Binding species are those that bond to biomolecules, altering their function in a detrimental way.
This binding may be reversible, as is the case with the binding of carbon monoxide with hemoglobin
(see Chapter 11), which deprives hemoglobin of its ability to attach molecular O2 and carry it from
the lungs to body tissues. The binding may be irreversible. An example is that which occurs when
an electron-deficient carbonium ion, such as H3C+ (an electrophile), binds to a nucleophile, such
as an N atom on guanine attached to deoxyribonucleic acid (DNA).
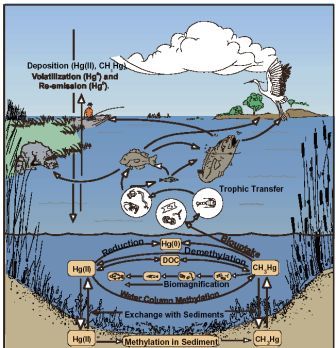
• Lipid-soluble compounds are frequently toxic because of their ability to traverse cell membranes
and similar barriers in the body. Lipid-soluble species frequently accumulate to toxic levels through
biouptake and biomagnification processes (see Chapter 5).
• Chemical species that induce a toxic response based largely on their chemical structures. Such
toxicants often produce an allergic reaction as the body’s immune system recognizes the foreign
agent, causing an immune system response. Lower-molecular-mass substances that act in this way
usually must become bound to endogenous proteins to form a large enough species to induce an
allergic response.
Biochemical Transformations
The toxicological chemistry of toxicants is strongly tied to their metabolic reactions and fates
in the body.1 Systemic poisons in the body undergo (1) biochemical reactions through which they
have a toxic effect, and (2) biochemical processes that increase or reduce their toxicities, or change
toxicants to forms that are readily eliminated from the body. In dealing with xenobiotic compounds,
the body metabolizes them in ways that usually reduce toxicity and facilitate removal of the
substance from the body, a process generally called detoxication. The opposite process by which
nontoxic substances are metabolized to toxic ones or by which toxicities are increased by biochemical
reactions is called toxication or activation. Most of the processes by which xenobiotic
substances are handled in living organisms are phase I and phase II reactions discussed in the
remainder of this chapter.
Metabolic Reactions Of Xenobiotic Compounds
Toxicants or their metabolic precursors (protoxicants) may undergo absorption, metabolism,
temporary storage, distribution, or excretion, as illustrated in Figure 7.1.2 The modeling and mathematical
description of these aspects as a function of time is called toxicokinetics.3 Here are
discussed the metabolic processes that toxicants undergo. Emphasis is placed on xenobiotic compounds,
on chemical aspects, and on processes that lead to products that can be eliminated from
the organism. Of particular importance is intermediary xenobiotic metabolism, which results in
the formation of somewhat transient species that are different from both those ingested and the
ultimate product that is excreted. These species may have significant toxicological effects. Xenobiotic
compounds in general are acted on by enzymes that function on an endogenous substrate
that is in the body naturally. For example, flavin-containing monooxygenase enzyme acts on
endogenous cysteamine to convert it to cystamine, but also functions to oxidize xenobiotic nitrogen
and sulfur compounds.
Biotransformation refers to changes in xenobiotic compounds as a result of enzyme action.
Reactions not mediated by enzymes may also be important. As examples of nonenzymatic transformations,
some xenobiotic compounds bond with endogenous biochemical species without an
enzyme catalyst, undergo hydrolysis in body fluid media, or undergo oxidation–reduction processes.
However, the metabolic phase I and phase II reactions of xenobiotics discussed here are enzymatic.
The likelihood that a xenobiotic species will undergo enzymatic metabolism in the body depends
on the chemical nature of the species. Compounds with a high degree of polarity, such as relatively
ionizable carboxylic acids, are less likely to enter the body system and, when they do, tend to be
quickly excreted. Therefore, such compounds are unavailable, or available for only a short time,
for enzymatic metabolism. Volatile compounds, such as dichloromethane or diethylether, areexpelled so quickly from the lungs that enzymatic metabolism is less likely. This leaves as the most
likely candidates for enzymatic metabolic reactions nonpolar lipophilic compounds, those that
are relatively less soluble in aqueous biological fluids and more attracted to lipid species. Of these,
the ones that are resistant to enzymatic attack (polychlorinated biphenyls (PCBs), for example)
tend to bioaccumlate in lipid tissue.
Xenobiotic species may be metabolized in a wide variety of body tissues and organs. As part
of the body’s defense against the entry of xenobiotic species, the most prominent sites of xenobiotic
metabolism are those associated with entry into the body (see Figure 6.2). The skin is one such
organ, as is the lung. The gut wall through which xenobiotic species enter the body from the
gastrointestinal tract is also a site of significant xenobiotic compound metabolism. The liver is of
particular significance because materials entering systemic circulation from the gastrointestinal tract must first traverse the liver.
Biodegredation of chemicals
Decay may be due to such processes as mineralization, detoxication, cometabolism, and microbial decay. The rate of loss of mass at any given time is directly proportional to the mass present at that time, the rate coefficients can be combined into a single decay coefficient.
∂CT =kbio CT
Where kb is overall biodegradation rate [1/T].
Absorption is the process whereby toxicants gain entrance to the body. Ingested and inhaled materials, nonetheless, are considered outside the body until they cross the cellular barriers of the gastrointestinal tract or the respiratory system. To exert an effect on internal organs a toxicant must be absorbed, although such local toxicity as irritation, may occur.
Absorption varies greatly with specific chemicals and with the route of exposure. For skin, oral or respiratory exposure, the exposure dose (or, "outside" dose) is usually only a fraction of the absorbed dose (that is, the internal dose). For substances injected or implanted directly into the body, exposure dose is the same as the absorbed or internal dose.
Several factors affect the likelihood that a foreign chemical or, xenobiotic, will be absorbed. The most important are:
route of exposure;
concentration of the substance at the site of contact; and
chemical and physical properties of the substance.
The relative roles of concentration and properties of the substance vary with the route of exposure. In some cases, a high percentage of a substance may not be absorbed from one route whereas a low amount may be absorbed via another route. For example, very little DDT powder will penetrate the skin whereas a high percentage will be absorbed when it is swallowed. Due to such route-specific differences in absorption, xenobiotics are often ranked for hazard in accordance with the route of exposure. A substance may be categorized as relatively non-toxic by one route and highly toxic via another route.
The primary routes of exposure by which xenobiotics can gain entry into the body are:
Gastrointestal tract
Important for environmental exposure to food and water contaminants. In addition, the main route for many pharmaceuticals.
Respiratory tract
Important for environmental and occupational exposure to air contaminants. Some pharmaceutical utilize this route.
Skin
Important for environmental and occupational exposure route. Many consumer and pharmaceutical products are applied directly to the skin.
Fig: Routes of exposure
Some toxicants can not simply diffuse across the membrane. They require assistance that is facilitated by specialized transport mechanisms. The primary types of specialized transport mechanisms are:
facilitated diffusion;
active transport; and
endocytosis (phagocytosis and pinocytosis).
Passive transfer is the most common way that xenobiotics cross cell membranes. Two factors determine the rate of passive transfer:
differences in concentrations of the substance on opposite sides of the membrane (substance moves from a region of high concentration to one having a lower concentration. Diffusion will continue until the concentration is equal on both sides of the membrane); and
ability of the substance to move either through the small pores in the membrane or through the lipophilic interior of the membrane.
Properties of the chemical substance that affect its ability for passive transfer are:
lipid solubility;
molecular size; and
degree of ionization (that is, the electrical charge of an atom).